Wireless device trialled in young patients to improve remote monitoring and diagnosis of sleep and respiratory conditions
A new wireless device that could help in the diagnosis of respiratory and sleep conditions in babies and young children is being trialled for the first time with patients.
The Paediatric Advanced Respiratory Service (PARS) has been developed by the West of Scotland Innovation Hub, which is hosted by NHS Greater Glasgow and Clyde, in partnership with digital therapeutics company PneumoWave.
It combines wireless wearable biosensors with mobile app-based software to give real-time analysis of infants’ breathing when they are asleep.
The technology is attached using an adhesive pad and tailored specifically for children, as many existing in-hospital devices are invasive and therefore poorly tolerated by babies and toddlers.
It will also be used to monitor babies in the Royal Hospital for Children’s Neonatal Unit in Glasgow who are at risk of central apnea.
Currently available for research use only, development of the technology in the PARS project has the potential to improve remote monitoring and diagnostic accuracy in conditions such as sleep disordered breathing, paediatric respiratory disease and acute neonatal compromise.
It is hoped that it will improve patient outcomes and experience, while also adding capacity to clinical services.
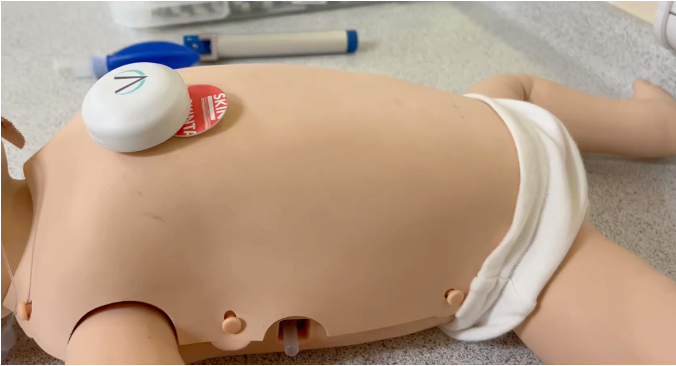
The device and software is now being used in an early stage study with 150 young patients from the Royal Hospital for Children - 75 from the Sleep Clinic Unit and 75 from the Neonatal Unit.
The study, which will run until October 2025, will allow medics to explore how tolerable it is and its feasibility going forward.
Dr Ross Langley, NHSGGC Paediatric Respiratory Consultant, has led the PARS project.
He said: “Detecting and monitoring respiratory problems in children and newborns can be challenging and we know that existing in-hospital devices are invasive and poorly tolerated by children.
“We are pleased that PARS has now entered an early stage study with patients as we explore new ways to diagnose sleep and respiratory conditions in young children.
“This has been developed with industry partner PneumoWave and is tailored specifically for children.
“The device has worldwide potential to improve remote monitoring and diagnostic accuracy in paediatric patients.”
Dr Bruce Henderson, CEO of PneumoWave, said: “Our goal as a company is to stop early deaths from preventable causes, and there is nothing more important than achieving this in children.
“We are privileged to have the support of patients at the Royal Hospital for Children, and of the dedicated staff led by Dr Langley, and grateful to Innovate UK for funding the project.”
The research infrastructure underpinning the study has been supported by Glasgow Children's Hospital Charity. CEO Kirsten Watson said: “We are delighted that our long-term investment in respiratory research at the children's hospital has enabled this exciting new development.
“It is crucial that we continue to innovate with minimally invasive technology designed specifically for our most vulnerable babies and children.
“Our heartfelt thanks go to the Reid-Timoney Charitable Foundation for their generous support of our three-year respiratory research strategy.”